
Top Performing Drug – Ibrance (August Edition)
Shots:
- In continuation of our previous series on the top-performing drug of the month, based on 2021 revenue, this month we have selected Ibrance and prepared a curated analysis report for our readers
- Ibrance is a targeted cancer therapy developed to treat certain types of advanced breast cancer. It belongs to a class of drugs called CDK4/6 inhibitors that work by blocking specific proteins involved in cell division
- PharmaShots presents a concise take on the key features of Ibrance with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: palbociclib
Dosage Forms & Strengths: Capsules (125 mg, 100 mg, and 75 mg)
Mechanism of Action: cyclin-dependent kinases (CDK) 4 and 6 inhibitors
Originators: Pfizer
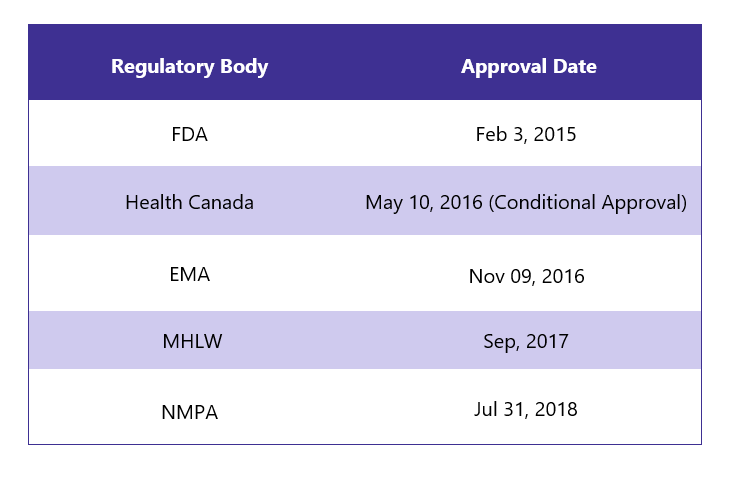
First Approvals: The table below depicts the first approvals of Ibrance from different regulatory agencies.
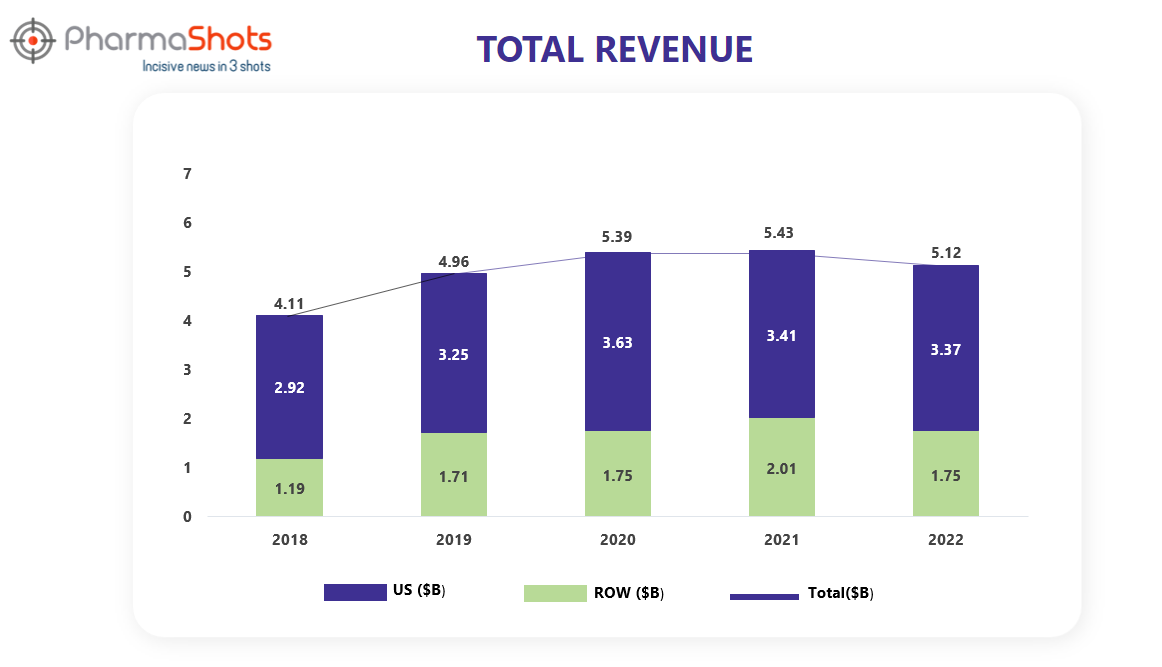
Revenue Analysis1
Ibrance was discovered and marketed by Pfizer. Ibrance was approved and launched in the U.S., Macau, Chile, and Albania as a first-line treatment for certain forms of advanced breast cancer. Ibrance generated global revenues of $723 million in 2015, with most of this revenue originating from the United States.
The analysis of Ibrance’s past 5 years’ revenue is showcased below through a graphical representation.
Ibrance Approved Indications2
Ibrance is a kinase inhibitor indicated for the treatment of adult patients with hormone receptor (HR)-positive, HER2-negative advanced, or metastatic breast cancer in combination with:
• an aromatase inhibitor as initial endocrine-based therapy
• fulvestrant in patients with disease progression following endocrine therapy
Clinical Trials Analysis3
Ibrance underwent 361 trials, incl. 216 industry trials, 181 of which were interventional, 33 observational, and 2 expanded access trials. (Trials were taken on 7th Aug 2023).
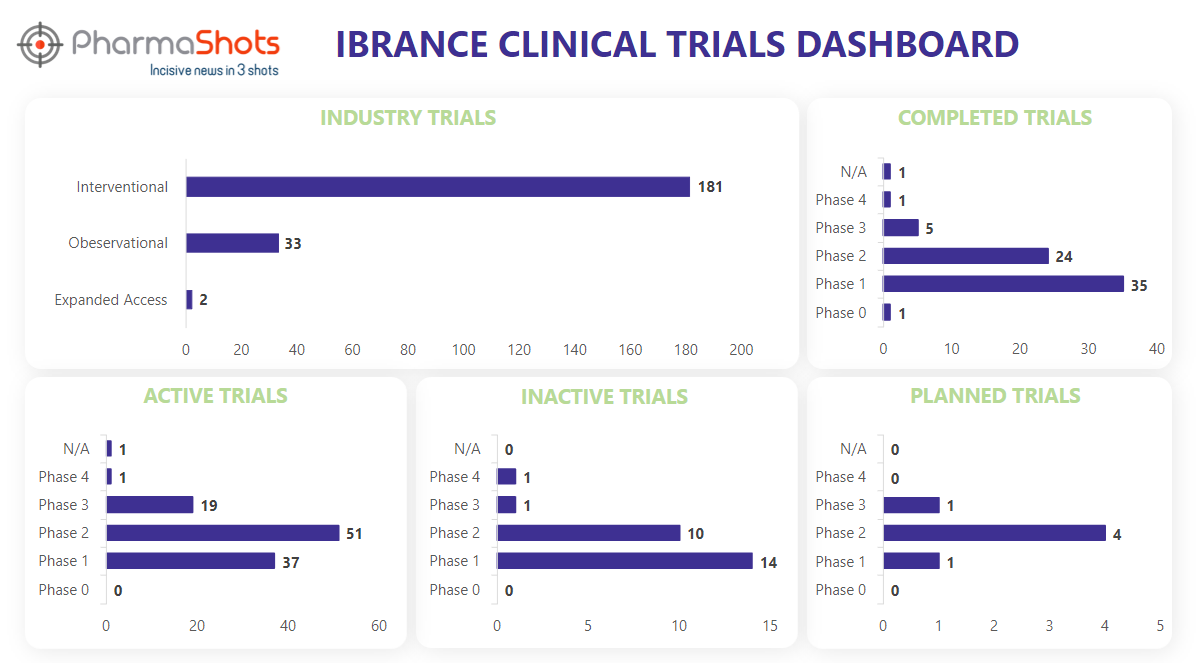
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Ibrance Trials Representation (Country-wise)4
The stacked column chart below is a visual representation of the ongoing evaluation of Ibrance in various indications worldwide. It focuses on the top 10 countries globally and includes only interventional studies.
* The chart depicts data till 11th Aug 2023
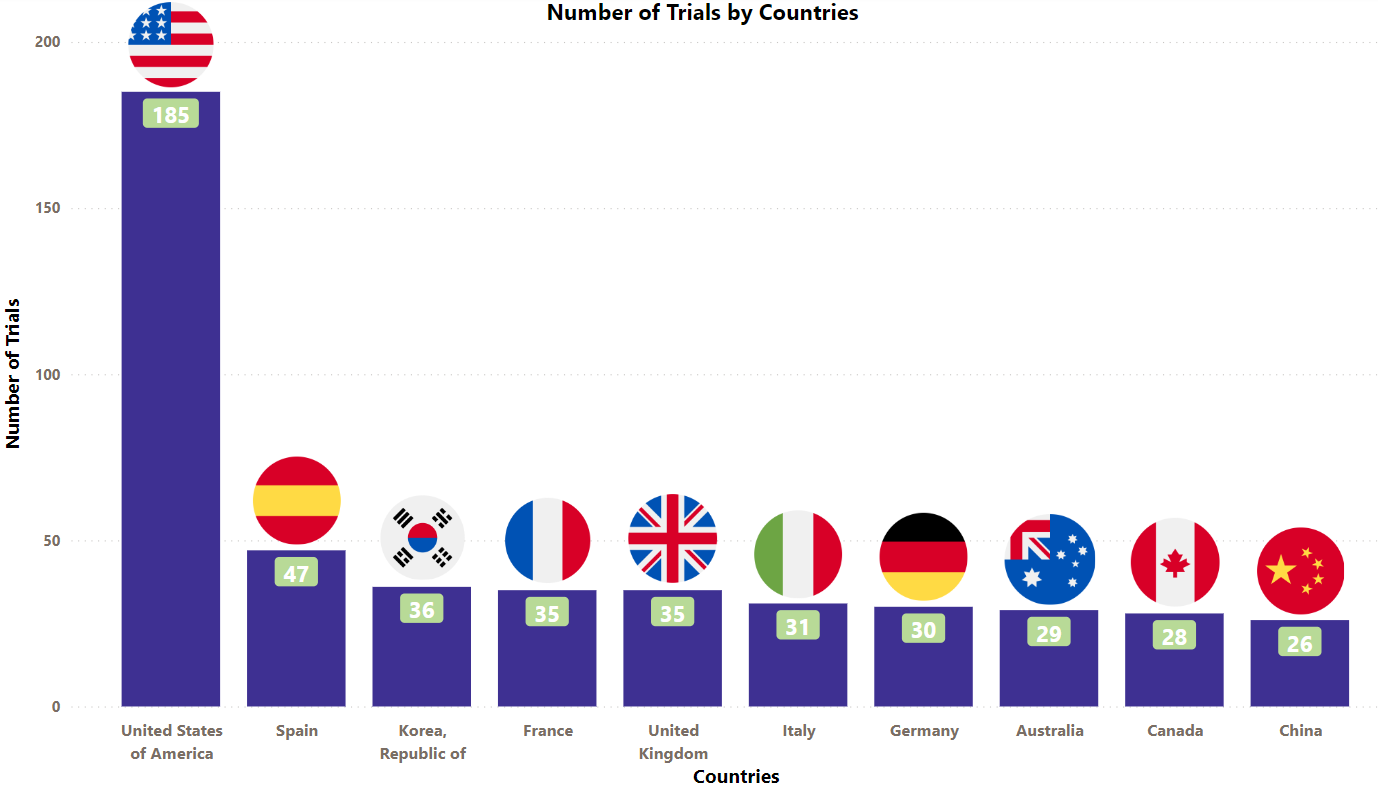
Besides the data shown in the chart, 12 countries have double-digit trial numbers, and 43 countries have single-digit trial numbers. For a detailed report on it, mail us at connect@pharmashots.com
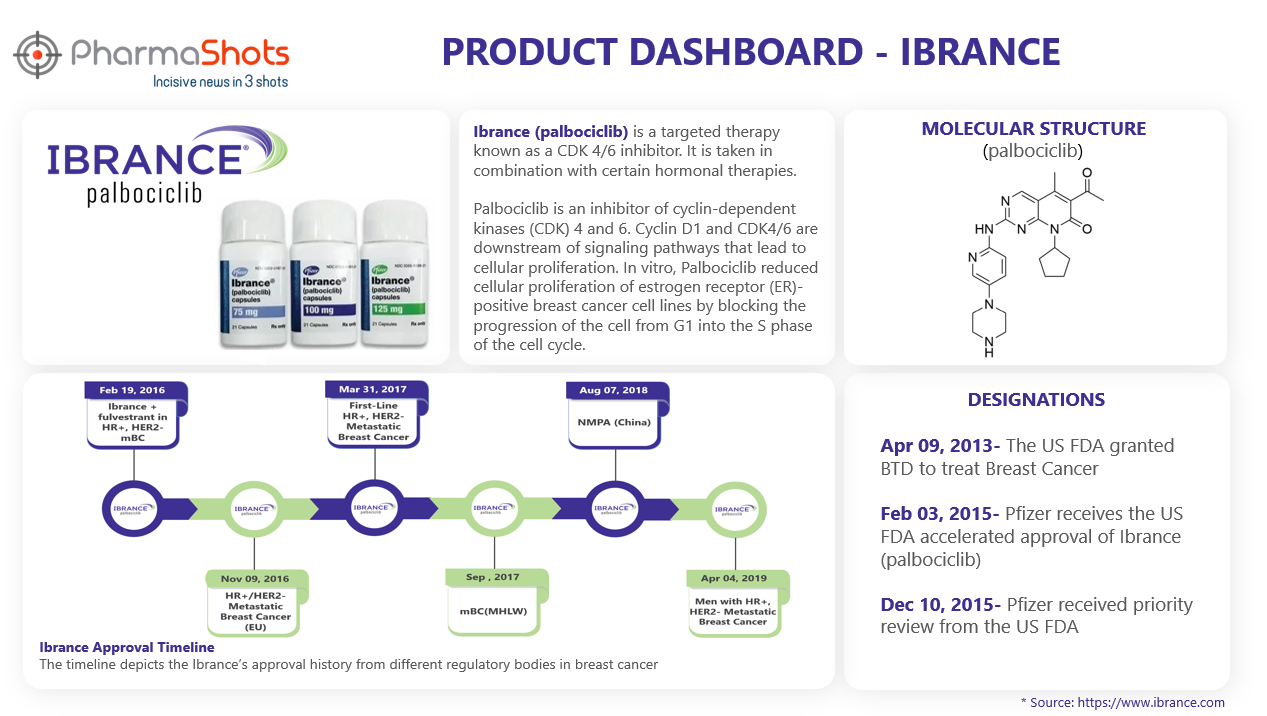
Product Dashboard
PharmaShots brings an illustrative infographic showcasing essential metrics and relevant data on Ibrance.
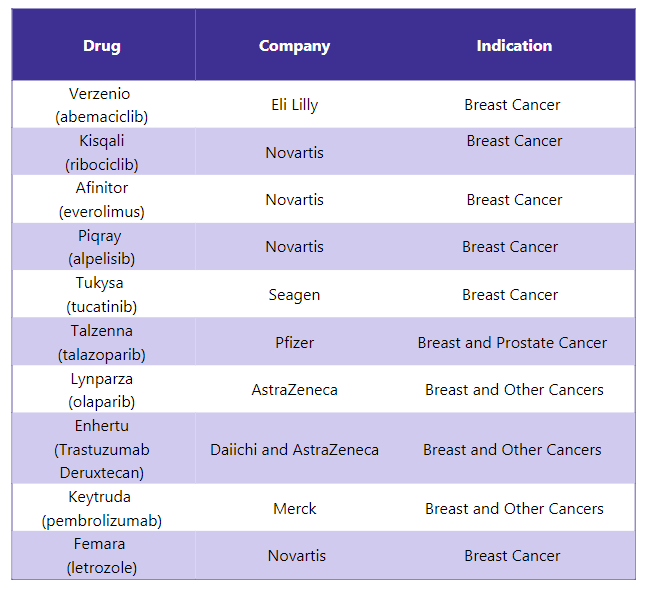
Ibrance Alternative Drugs5
In response to Ibrance, several other drugs are available in the market and are used to treat different indications. Some of the alternative drugs for Ibrance include:
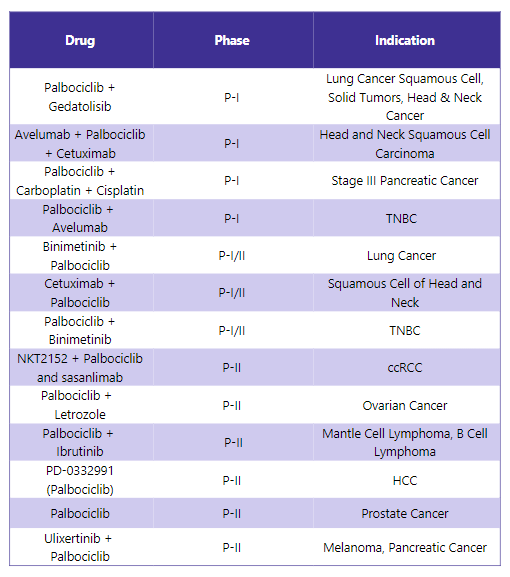
Ibrance Pipeline Analysis6
PharmaShots presents a thorough analysis of the Ibrance pipeline, including ongoing Phase I, I/II, and Phase II registration studies for various indications. The table shown below is an overview of the status of these studies
Ibrance SWOT Analysis7
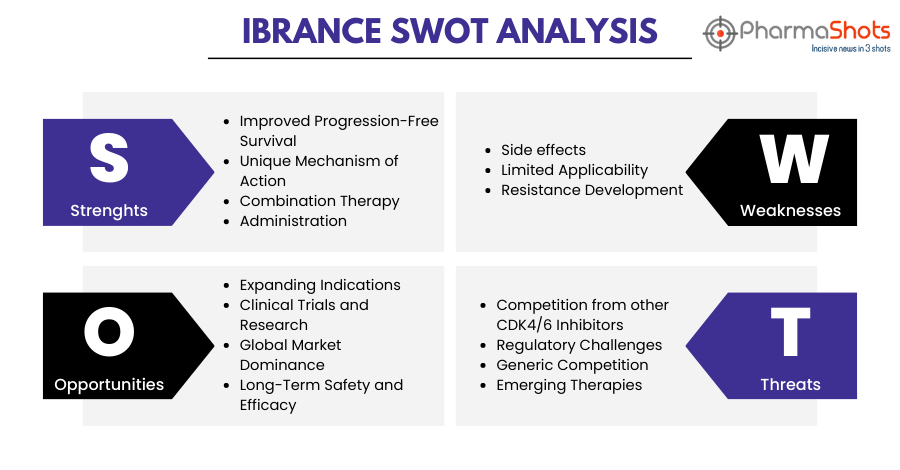
Strengths:
- Improved Progression-Free Survival: A clinical study has shown that adding Ibrance to hormone therapy Faslodex in postmenopausal women with HR-positive, HER2-negative advanced breast cancer can lead to improved progression-free survival compared to Faslodex alone
- Unique Mechanism of Action: Ibrance is a targeted therapy that specifically inhibits CDK4/6. This MoA is unique and helps control the uncontrolled growth of cancer cells, which can move to different areas of the body
- Combination Therapy: Ibrance is often used in combination with other hormonal therapies like aromatase inhibitors, enhancing its effectiveness. When combined with an aromatase inhibitor, letrozole, the combination delayed disease progression for a median time of 24.8 months versus 14.5 months and reduced their risk of disease progression by 42% for those that received letrozole and a placebo
- Administration: Ibrance is available in an oral form, usually taken as a capsule, which allows patients to take the medication at home. This can provide convenience and flexibility in managing their treatment regimen
Weaknesses:
- Side effects: Ibrance can cause certain side effects, some of which are more common than others. Some of its side effects include neutropenia (low white blood cell count), infections, tiredness, nausea, sore mouth, abnormalities in liver blood tests, diarrhea, hair thinning or hair loss, vomiting, rash, and loss of appetite. These side effects can impact a patient's quality of life and may require dose adjustments or treatment interruptions
- Limited Applicability: Ibrance is used for the treatment of certain types of (HR+/HER2-) advanced or metastatic breast cancer. This restricts its use to a subset of breast cancer patients, limiting its potential market
- Resistance Development: Over time, some patients may develop resistance to Ibrance, leading to reduced treatment effectiveness. This poses a challenge in maintaining long-term patient outcomes
Opportunities:
- Expanding Indications: There could be opportunities to explore the potential of Ibrance in treating other types of cancers with similar mechanisms of action or molecular pathways
- Clinical Trials and Research: Continued research into Ibrance and its mechanisms of action could lead to the identification of new indications, combination therapies, or ways to mitigate resistance
- Global Market Dominance: As awareness and diagnosis of breast cancer continue to improve globally, the market for targeted therapies like Ibrance could expand, particularly in emerging markets
- Long-Term Safety and Efficacy: Continued research can provide more insights into the long-term safety and efficacy of Ibrance improving the medication's impact on survival rates and quality of life
Threats:
- Competition from other CDK4/6 Inhibitors: Ibrance faces competition from other CDK4/6 inhibitors like Kisqali, Verzenio, etc. in the market. This could impact market share and pricing strategies
- Regulatory Challenges: Evolving regulatory requirements and new reforms could impact the approval process and reimbursement landscape for Ibrance
- Generic Competition: The expiration of the patent for Ibrance could lead to the entry of generic versions, potentially reducing the drug’s market share and profitability
- Emerging Therapies: The development of new and potentially more effective therapies for advanced breast cancer could pose a threat to Ibrance's market position
Patient Stories8
Patients' stories have been instrumental in sharing their perspectives on individual experiences in facing health challenges. These are a few key resources to understand healthcare services and policies. Some of the patients’ stories for Ibrance are mentioned below:
Jami’s Story: After the diagnosis of stage I breast cancer, within two months, Jami had gone to stage IV metastatic breast cancer and then her oncologist suggested initiating treatment with Ibrance + letrozole within 1 to 2 weeks her insurance got approved. She said: “At my next oncologist visit, we started talking about my treatment. He wanted me to begin an oral medication called IBRANCE”. Read Jami’s full story.
Norma’s Story: Norma said that she did not have any indication of cancer for 14 years, and after that, her cancer came back with a stage IV diagnosis. She mentioned that this Stage IV diagnosis was much worse than her initial breast cancer diagnosis. She said: “None of us know what our future is—no matter what we’re dealing with, the best we can do, I think, is trying to look for new adventures. Today, I am grateful for the ongoing medical advances in metastatic breast cancer. I am grateful for life.” Read Norma’s full story.
Cindy’s Story: Cindy was diagnosed with breast cancer for the first time in May 2007. Nearly a decade after her first diagnosis of early-stage breast cancer and a double mastectomy, Cindy received the news that she had mBC. She said: “My doctor told me about IBRANCE, a first-line treatment option for postmenopausal women with HR+/HER2- metastatic breast cancer, taken in combination with letrozole—which is an aromatase inhibitor. I knew I had to have faith in my treatment plan and hope for the best.” Read Cindy’s full story
KOL* Reviews9
KOL reviews are valuable resources for any drug to increase its reach and reliability. Generally, these reviews are helpful when consumers research the product and read multiple reviews before buying it. Below are some of the KOL reviews for Ibrance.
In Apr 2019, the US FDA approved Ibrance for the treatment of men with HR+, HER2- metastatic breast cancer.
- Bret Miller, Founder of the Male Breast Cancer Coalition said that “Men with breast 22 have limited treatment options, making access to medicines such as IBRANCE critically important. We applaud the use of real-world data, a new approach to drug review, to make IBRANCE available to certain men with metastatic breast cancer and help address an unmet need for these patients.”
- Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence said: “Today we are expanding the indication for Ibrance to include male patients based upon data from postmarketing reports and electronic health records showing that the safety profile for men treated with Ibrance is consistent with the safety profile in women treated with Ibrance.”
In Jun 2022, Pfizer reported Overall Survival results from the P-III study (PALOMA-2) of Ibrance (palbociclib) for the 1L Treatment of ER+, HER2- metastatic breast cancer.
- Chris Boshoff, M.D., Ph.D., Chief Development Officer, Oncology, Pfizer Global Product Development said: “IBRANCE continues to provide substantial benefit as a first-line treatment for adults with HR+, HER2- mBC based on strong progression-free survival data, which formed the basis of its worldwide approvals.”
- Richard Finn, M.D., Professor of Medicine at UCLA said: “IBRANCE transformed the treatment landscape for patients with HR+, HER2- MBC when it was approved in 2015, representing the first new treatment in this patient population in over a decade.”
In Oct 2015, Prime Therapeutics presented a new study at the Academy of Managed Care Pharmacy (AMCP) Nexus event on the utilization and cost of Ibrance which earned a gold status for quality research at the Academy of Managed Care Pharmacy (AMCP) Nexus event.
- Pat Gleason, PharmD, director of health outcomes at Prime, said: "As the FDA is approving cancer drugs more quickly and with limited safety data, it is essential for insurers to do surveillance studies – like this with Ibrance – so we can ensure our members are safely and effectively getting the medicine they need to feel better and live well. With the Ibrance safety concerns and a $476 per capsule cost, it is vital that we work with our health plan clients to assess and implement effective programs that address appropriate use and prevent unnecessary waste and costs."
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
2. Ibrance Prescribing Information
4. Ibrance Trials (Country-wise)
5. Ibrance Alternative Drugs
7. SWOT Analysis
9. KOL Reviews
Related Post: Top Performing Drug – Ocrevus (July Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














